During 1980s Bowling Barnes, David Richardson, John Berry and Robert Hood developed an instrument to measure the low concentrations of sodium and potassium in a solution. They named this instrument as Flame photometer. The principle of flame photometer is based on the measurement of the emitted light intensity when a metal is introduced into the flame. The wavelength of the colour gives information about the element and the colour of the flame gives information about the amount of the element present in the sample.
Flame photometry is one of the branches of atomic absorption spectroscopy. It is also known as flame emission spectroscopy. Currently, it has become a necessary tool in the field of analytical chemistry. Flame photometer can be used to determine the concentration of certain metal ions like sodium, potassium, lithium, calcium and cesium etc. In flame photometer spectra the metal ions are used in the form of atoms. The International Union of Pure and Applied Chemistry (IUPAC) Committee on Spectroscopic Nomenclature has named this technique as flame atomic emission spectrometry (FAES).
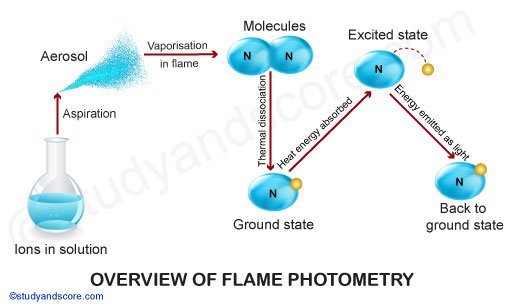
The compounds of the alkali and alkaline earth metals (Group II) dissociate into atoms when introduced into the flame. Some of these atoms further get excited to even higher levels. But these atoms are not stable at higher levels.
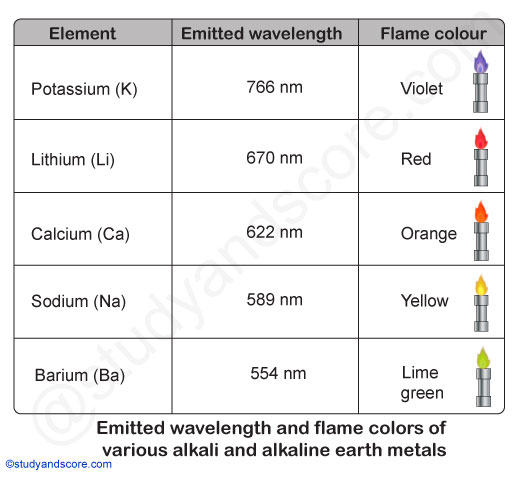
Hence, these atoms emit radiations when returning back to the ground state. These radiations generally lie in the visible region of the spectrum. Each of the alkali and alkaline earth metals has a specific wavelength.
| Element | Emitted wavelength | Flame color |
|---|---|---|
| Sodium | 589 nm | Yellow |
| Potassium | 766 nm | Violet |
| Barium | 554 nm | Lime green |
| Calcium | 622 nm | Orange |
| Lithium | 670 nm | Red |
For certain concentration ranges,
The intensity of the emission is directly proportional to the number of atoms returning to the ground state. And the light emitted is in turn proportional to the concentration of the sample.
A simple flame photometer consists of the following basic components:
Source of flame: A Burner in the flame photometer is the source of flame. It can be maintained in at a constant temperature. The temperature of the flame is one of the critical factors in flame photometry.
| Fuel-Oxidant mixture | Temperature (°C) |
|---|---|
| Natural gas-Air | 1700 |
| Propane-Air | 1800 |
| Hydrogen-Air | 2000 |
| Hydrogen-Oxygen | 2650 |
| Acetylene-Air | 2300 |
| Acetylene-Oxyen | 3200 |
| Acetylene-Nitrous oxide | 2700 |
| Cyanogen-Oxygen | 4800 |
Nebuliser: Nebuliser is used to send homogeneous solution into the flame at a balanced rate.
Optical system: The optical system consists of convex mirror and convex lens. The convex mirror transmits the light emitted from the atoms. Convex mirror also helps to focus the emissions to the lens. The lens helps to focus the light on a point or slit.
Simple colour filters: The reflections from the mirror pass through the slit and reach the filters. Filters will isolate the wavelength to be measured from that of irrelevant emissions.
Photo-detector: The intensity of radiation emitted by the flame is measured by photo detector. Here the emitted radiation is converted to an electrical signal with the help of photo detector. These electrical signals are directly proportional to the intensity of light.
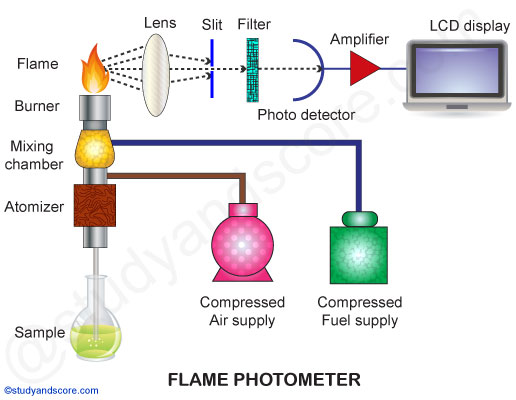
The oxidants in flame photometer are mainly air, oxygen or nitrous oxide. The temperature of the flame depends on the ratio of fuel and oxidant.
The processes occurring during flame photometer analysis are summarized below:
Desolvation: Desolvation involves drying a sample in a solution. The metal particles in the solvent are dehydrated by the flame and thus solvent is evaporated.
Vaporization: The metal particles in the sample are also dehydrated. This also led to the evaporation of the solvent.
Atomization: Atomization is the separation of all atoms in a chemical substance. The metal ions in the sample are reduced to metal atoms by the flame.
Excitation: The electrostatic force of attraction between the electrons and nucleus of the atom helps them to absorb a particular amount of energy. The atoms then jump to the higher energy state when excited.
Emission: Since the higher energy state is unstable the atoms jump back to the ground state or low energy state to gain stability. This jumping of atoms emits radiation with characteristic wavelength. The radiation is measured by the photo detector.
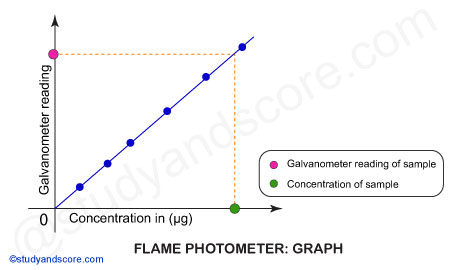
Scheibe-Lomakin equation describes intensity of light emitted with the help of following formula:
I = k × cn
Where:
I = Intensity of emitted light
c = Concentration of the element
k = Proportionality constant
At the linear part of the calibration curve n~1,
then I = k × c. In other words, the intensity of emitted light is directly related to the concentration of the sample.
- Share with your friends! -
Saud Malik on Mar 15, 2018 at 08:10 pm
Its very informative.... i prepared this topic for exam...My paper is on monday ..pray for me ..nd thanx for such a extraordinary topic


Himabindu Malla on Jun 29, 2018 at 06:00 pm
The manner in which the article is written and explained by using a diagram is awesome and very helpful.

Study Score on Jul 02, 2018 at 10:17 am
Thank you Himabindu. Do explore all other articles on our website.
Afaq Basra on Dec 02, 2018 at 09:25 am
hi, admin please share the name of the publisher/writer?

Login to post your comment here...
- or with social Account -